Flouride is the Solution
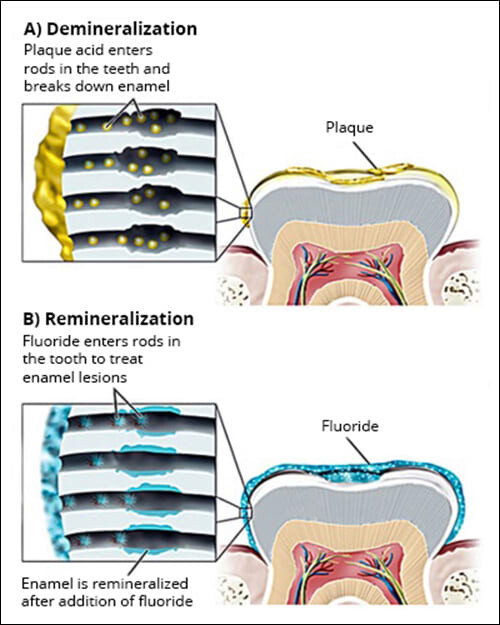
You often hear that fluoride is good for your teeth, but have probably not given it a second thought as to how it works. Fluoride, which is present in drinking water, toothpaste, mouthwashes, etc. , works by subtly altering the natural equilibrium of chemicals in your saliva and teeth. Your teeth contain the elements phosphate, and calcium, and a compound of these two called hydroxyapatite. In a natural process, phosphate and calcium in your teeth dissolve into your saliva and come into equilibrium. That is to say, if calcium in your teeth is lost, for instance through the action of bacteria and plaque, then calcium already dissolved and present naturally in your saliva can deposit itself back onto your teeth, a process called demineralization and remineralization. Bacteria and plaque pushes this equilibrium towards demineralization – calcium is lost from your teeth at a greater rate than it is deposited back. Fluoride helps to maintain the equilibrium of calcium and phosphate by combining with hydroxyapatite to form fluorapatite, which has the property of not dissolving as easily. Fluoride helps to keep your calcium and phosphate in your teeth and from dissolving/demineralizing, keeping them hard, strong, and therefore resistant to bacteria and plaque. These are specialized terms, but it is not hard to understand that while the body has beautiful mechanisms to maintain itself, a little scientific and proven intervention, such as the use of fluoride, goes a long way. Best wishes! – ProDentalFx, LLC. (Source: Dentalcare.com)